Entorhinal cortex
The rat entorhinal cortex (EC) makes up the ventrocaudal part of the rat cerebral hemisphere. The EC borders the parasubiculum (PaS), piriform cortex and the amygdaloid complex. Dorsolaterally it borders the rhinal fissure and rostrally it forms its fundus and dorsal bank.
Stratification
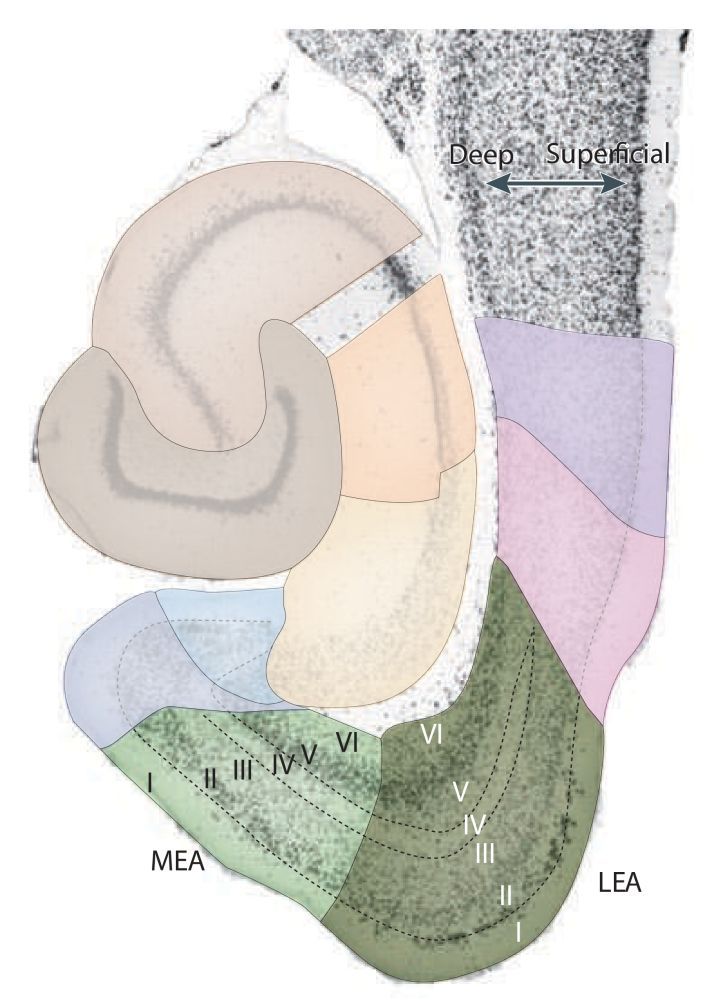
In this description of the stratification/lamination of the entorhinal cortex, the most superficial layer, close to the pial surface of the brain comes first, followed by the layers below. The EC consists of six layers, four cell layers (II, III, V and VI) and two plexiform layers (I, IV or lamina dissecans). Layers II and V are sometimes split into a superficial part 'a' and deep part 'b' (Haug, 1976 (p. 17)). The Swanson atlas (2018), plates 28 - 48, show the location of layers IIa / IIb, but does not divide layer V. There are approximately 675.000 principle neurons in the EC (Schmidt, 2012). Several accounts of the cell counts in the EC and its layers/divisions have been published (e.g. Mulders, 1997; Merrill, 2001; Rapp, 2002; Gatome, 2010). For computer modelers, a book dedicated to hippocampal microcircuits is available (see Witter, 2018). In the list below, each layer is briefly described (See: Cappaert, 2015 for more details).
-
I: Plexiform layer; sparsely populated by horizontal and multipolar neurons (Blackstad, 1956; Haug, 1976)
-
II: Cell layer; containing medium and big sized modified pyramidal and pyramidal-like cells generally organized in clusters (Lorente de Nó, 1933; Klink and Alonso, 1997, Insausti et al, 1997).
-
IIa: in lateral portions of the EC, an outer layer IIa and a deeper layer IIb can be seen, separated by a narrow cell-sparse layer Insausti et al, 1997. Depending on the parcellation scheme, layer II = Layer IIa. In that case, layer II is narrower and layer IIb is considered to be superficial layer III.
-
IIb: in lateral portions of the EC, an outer layer IIa and a deeper layer IIb can be seen, separated by a narrow cell-sparse layer Insausti et al, 1997. Depending on the parcellation scheme, superficial layer III = Layer IIb. If layer IIb is differentiated, then layer III is narrower.
-
III: Cell layer; contains predominantly pyramidal cells, but also other cells of various sizes and shapes (e.g. multipolar, fusiform, horizontal and bipolar cells).
-
IV: Plexiform layer; contains scattered cell bodies of various sizes and shapes (e.g. pyramidal, fusiform and bipolar cells) Lingenhohl and Finch, 1991). Here we use layer IV as a synonym for the 'lamina dissecans'. However, some authors describe the 'lamina dissecans' as a separate entity (See also: Haug, 1976 (p.11), Stephan, 1975 (pp. 206-211, 650-653, 661, 665, 694). In the nomenclature by Lorente de Nó, 1933, the 'lamina dissecans' is considered to be the deep part of layer III (see also layer Va).
-
V: Cell layer; contains pyramidal, horizontal and polymorphic neurons.
-
Va: a few cells wide in diameter; contains large pyramidal neurons that are unequally distributed along the extent of both MEC and LEC Insausti et al, 1997. In the nomenclature by Lorente de Nó, 1933, the current layer Va was termed layer IV.
-
Vb: a few rows of cells wide in diameter; contains smaller neurons compared to layer Va and are more densely packed. Insausti et al, 1997
-
VI: Cell layer; contains pyramidal(-like) and multipolar cells
Subdivisions
Several parcellation schemes exist for the entorhinal cortex. Brodmann (1909) was the first who parceled the EC into two fields: A lateral area 28a (or LEC) and a medial area 28b (or MEC). Subsequent descriptions were made by (a.o.) Stephan, 1975, Witter (2002, Insausti et al, 1997, Witter, 2017). Here we use the 2 subfield division, but several authors subdivided the EC into more than 2 subfields. These subdivisions delineate CE, ME, DIE, VIE, VLE and AE. AE was later found not to be part of the EC (see: Kemppainen et al., 2002; Majak and Pitkänen, 2003). We use the term LEC to include DLE, DIE, and VIE, whereas MEC includes CE and ME.
See the 6 subdivisions of the entorhinal cortex in the Hippocampus Atlas
Lateral and medial entorhinal cortex: differentiation
Some of the characteristic cytoarchitectonic differences between LEC and MEC are:
- LEC Layer II* is clearly demarcated; its cells are very densely packed and tend to be clustered in islands. The cells in MEC layer II are somewhat larger and show less of a distinct clustering into islands when viewed with a neuronal stain. However, the distribution of different celltypes in MEC do show a marked clustering in several species, including rat (Naumann et al, 2015, 2018).
- he border between layers II* and III in MEC is not as sharp as in the LEC, although in both entorhinal areas the overall difference in cell size between layers II and III facilitate the delineation of the two layers.
- The lamina dissecans of the MEC is sharply delineated but is less clear in the LEC. The other cell layers, in particular layers IV–VI, can be better differentiated from each other in the MEC than in the LEC, and cells in the deep layers of the MEC generally show a more radial or columnar arrangement.
In addition to cytoarchitecture as a criterion to subdivide the EC, one can look at its connectivity to define the LEC/MEC subfields. In the rat, LEC and MEC can also be distinguished by looking at their differential connectivity 1) to the molecular layer of the Dentate Gyrus (DG) and 2) to the differential connectivity along the proximodistal axis of hippocampal subfield CA1. Specifically:
- The LEC-> DG projection terminates in the outer one third (close to the pial surface) of the dentate molecular layer, whereas the MEC->DG projection terminates in the middle one-third of the dentate molecular layer (Hjorth-Simonsen and Jeune, 1972; Hjorth-Simonsen, 1972).
- The MEC-> CA1 projection terminates in the proximal part of CA1 (closer to the DG), whereas the LEC-> CA1 projection terminates in the distal part of CA1 (see also: van Strien et al., 2009).
Suggested reading
Canto, C.B. & Witter, M.P. (2012). Cellular properties of principal neurons in the rat entorhinal cortex. II. The medial entorhinal cortex. Hippocampus, 22(6), 1277-1299. Resolve DOI
References
Do you want to read more about the entorhinal cortex?
We have prepared a pubmed search for you to get started.